Covid-19 Roadmap - Spring 2021
Last week the Prime Minister set out the Government’s Roadmap out of lockdown.
Lockdown has been hugely difficult and damaging for so many households, families, pubs, shops and local businesses and communities. Having run my own small business for fifteen years in our area before being elected to Parliament, I absolutely understand and feel that pain.
I have been on the business frontline myself and have been doing everything possible over the last twelve months to help all those small businesses in our community that are struggling to make ends meet. The route out of lockdown provides hope to us all.
But, over the coming months, it is all the more vital that we all support local small businesses, the self-employed and the younger generation in every way we can.
In the short-term, that means vaccinations for all and continuing to follow the Government's rules. The vaccine rollout has rightly been a source of great national pride. We must continue to encourage everyone to take up the offer of a vaccine and bring down infections and case rates over the coming weeks. We must also continue to abide by the social distancing precautions. The stay-at-home order is still in place as is the guidance around hygiene, handwashing, mask-wearing and working from home if we can. We cannot let the virus resurge again. Compliance is still our best weapon against the virus. We must not let our guard down in this final phase.
Longer-term, however, it’s also clear that the structural damage and legacy of the Covid pandemic will require some big bold measures from Government to ensure a strong post-Covid Recovery.
We will need clear measures in four key areas:
1. First, training and recruitment support for the c50,000 forecast to lose their jobs when furloughing ends
2. Second, tax support for local small businesses to ‘bounce back’. We have 30,000 small businesses in Norfolk and they are the key to our economic recovery
3. Third, innovative approaches to get reinvestment and new energy into our High Streets and Town Centres which have been so badly damaged
4. Fourth, new thinking on how we can ‘Build Back Better’ and capture the public desire for healthier and cleaner living and working. Norfolk’s economic revival must be based around less commuting, congestion, pollution and more people able to work from or closer to home and work smarter and healthier.
—
If we get this right, the post-Covid Recovery is an opportunity to embrace an ambitious vision for Norfolk, as I set out in my recent article in the EDP: https://www.edp24.co.uk/news/unleash-norfolk-as-a-science-and-innovation-county-7565404
But this won’t just happen. It will need vision, leadership and partnership working. That’s why I’m working on a number of local projects to support this: the Norfolk Way project to promote Rural Enterprise; the Norfolk Enterprise Festival to bring our high growth small business leaders together to create new opportunities; and the Restart Project with the New Anglia LEP to set out a MicroBusiness Manifesto and a Norfolk Routemap to Net Zero plan for healthier growth.
The end of the Pandemic may be drawing near. But we then need to embark on a big bold Recovery Plan. In Norfolk, of Norfolk and by Norfolk.
This is an historic moment. Together we can rebuild the County we all love. Future generations will not forgive us if we fail.
The government has published the ‘COVID-19 Response - Spring 2021’, setting out the roadmap out of the current lockdown for England.

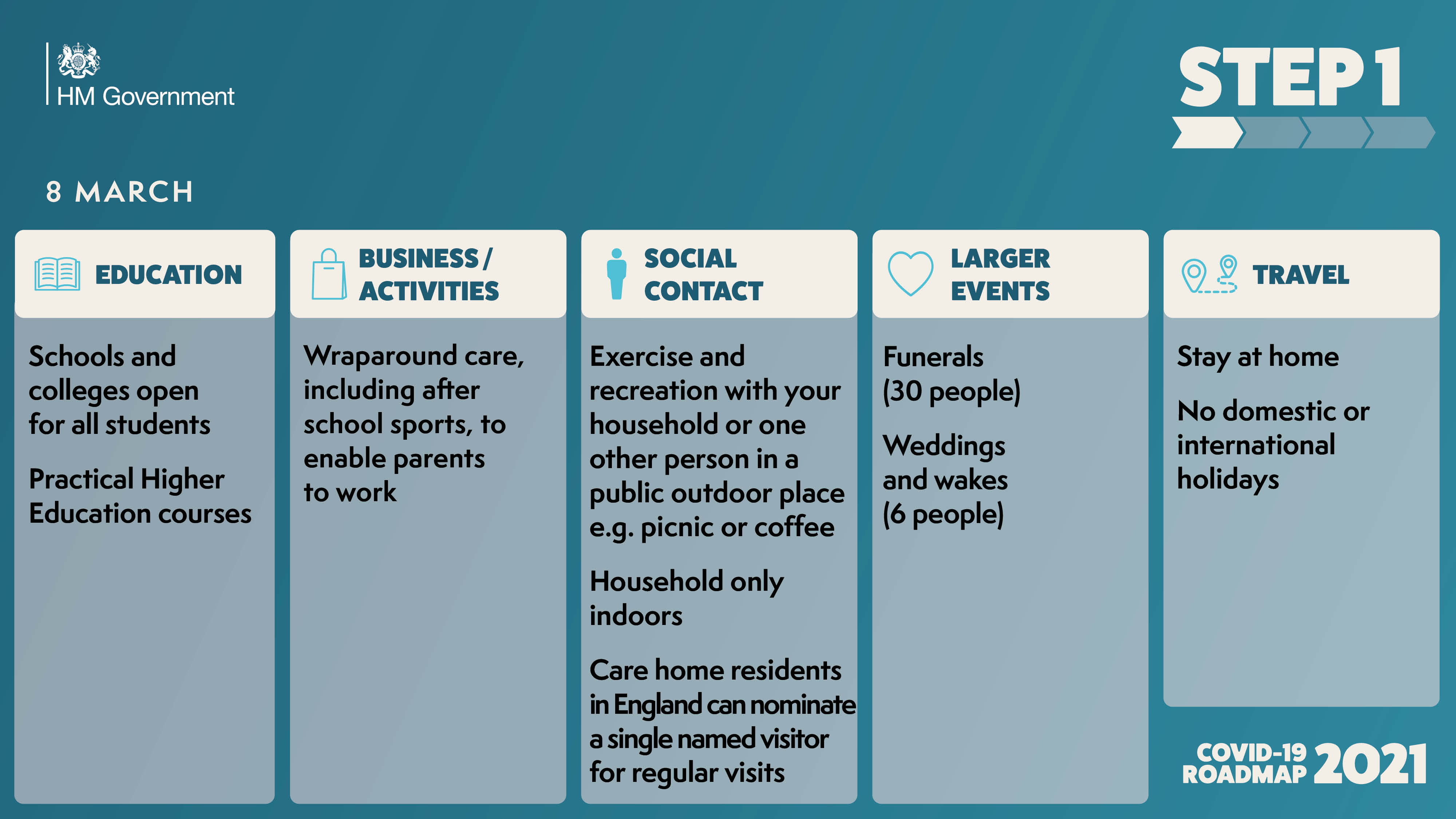
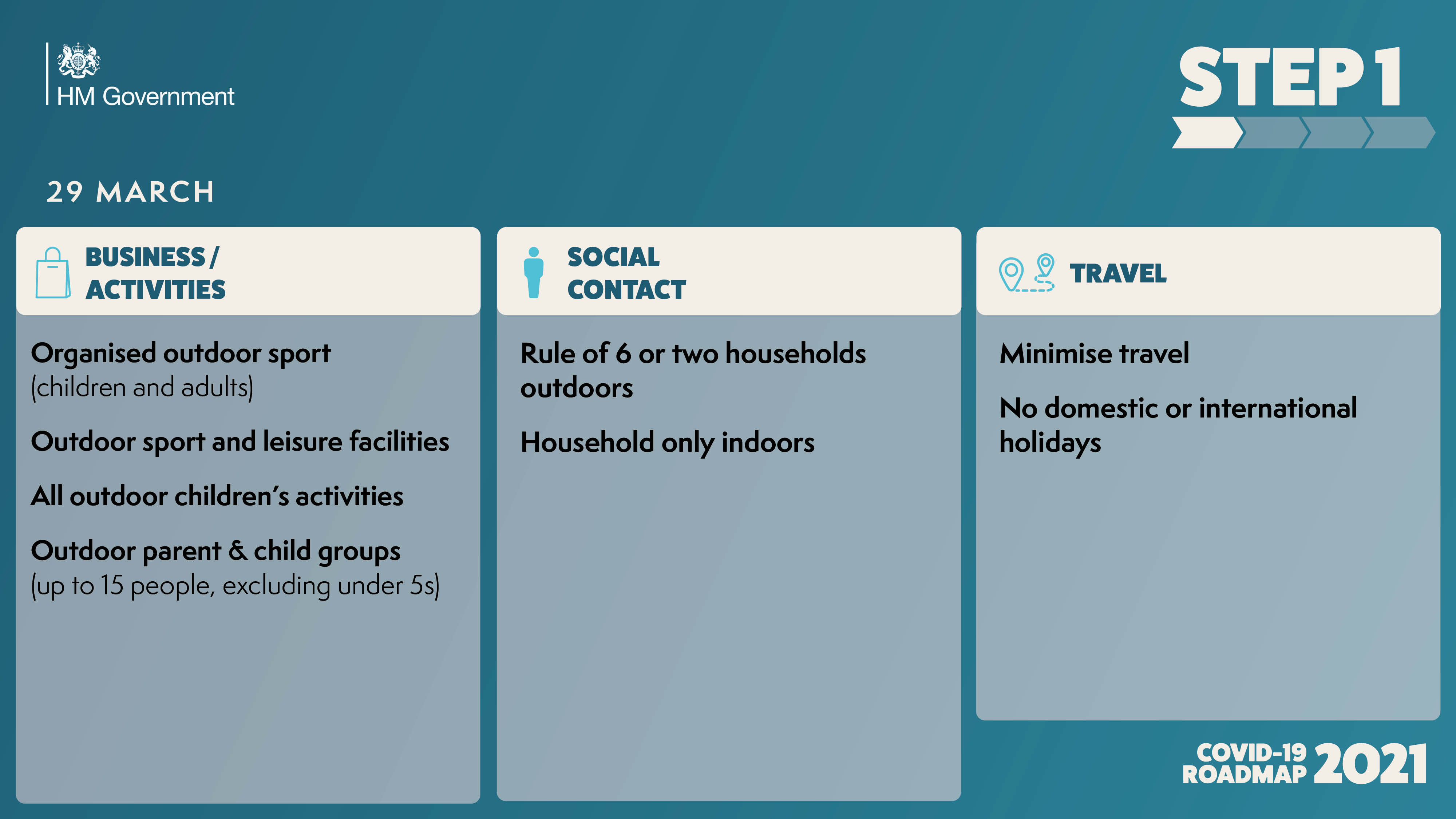
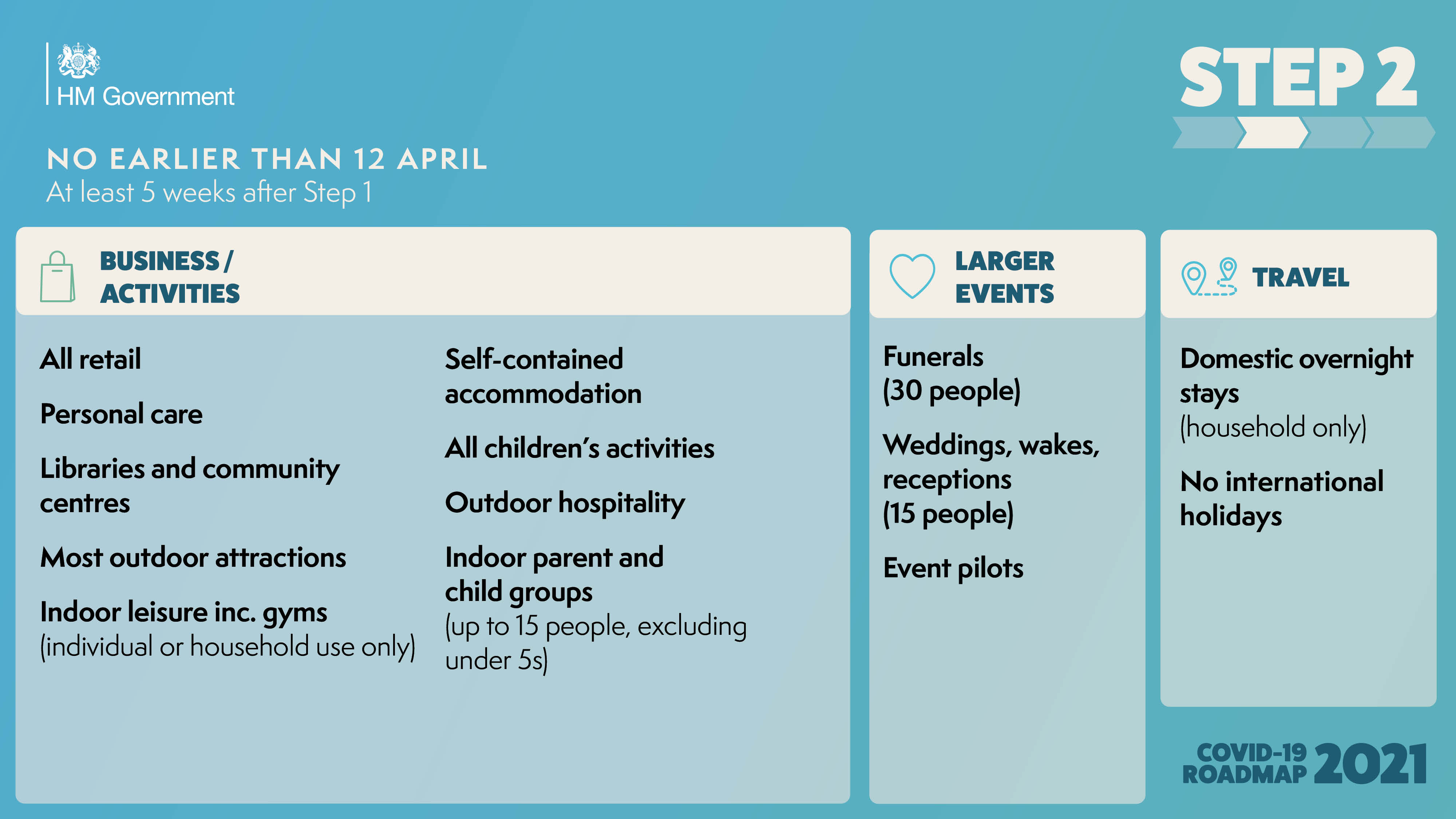
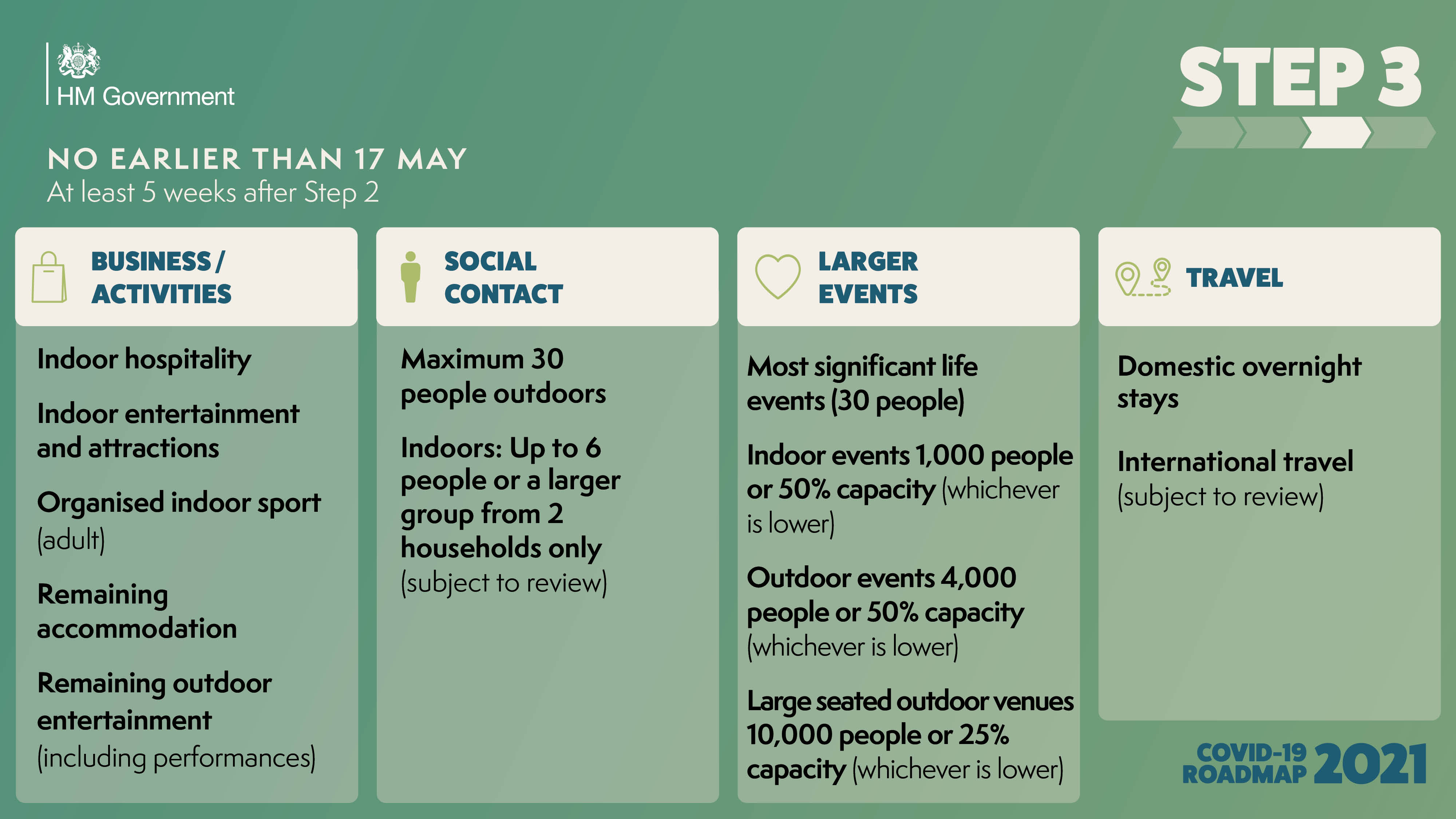
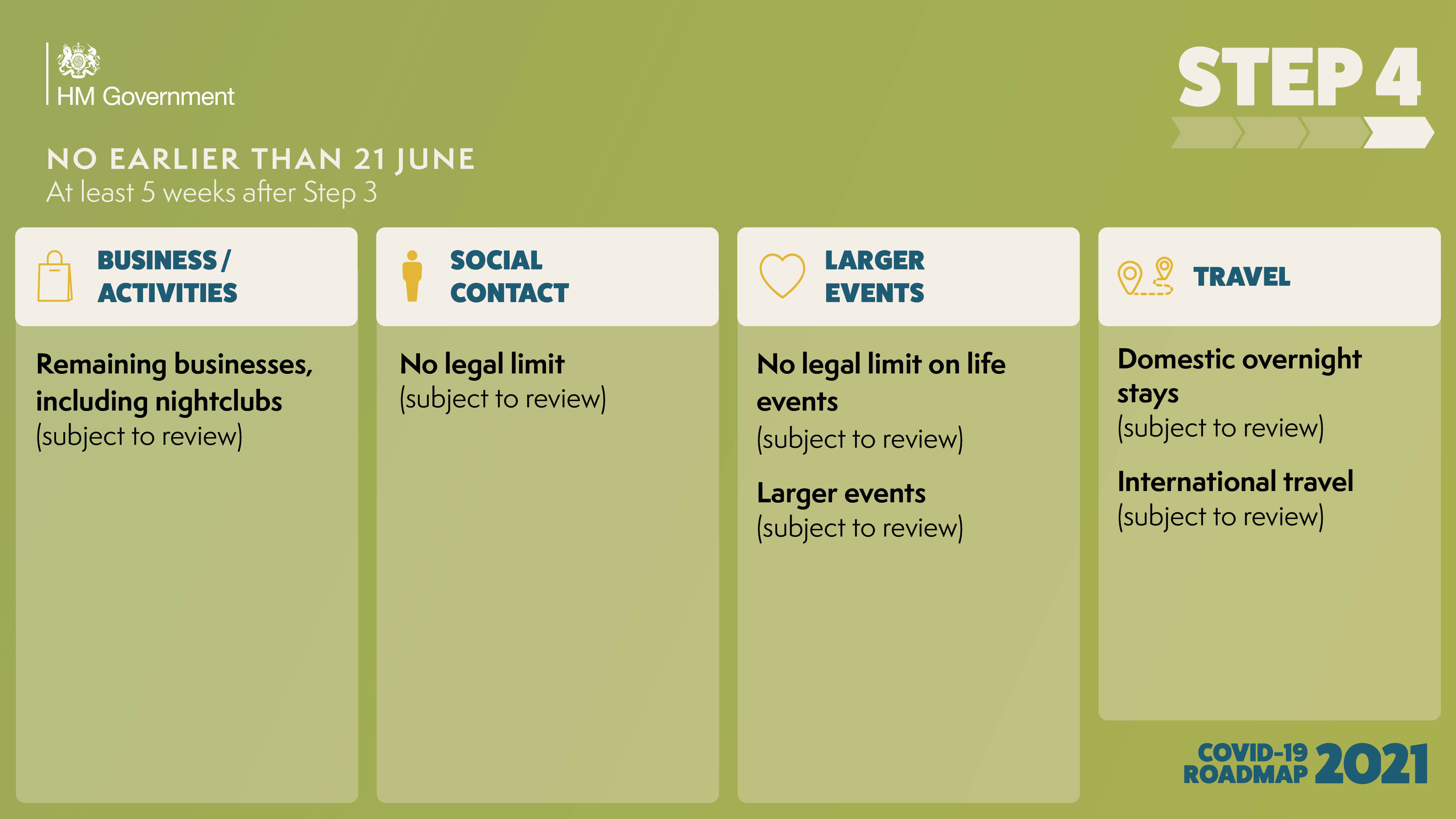
As the UK looks to embrace a greener, healthier Net Zero future, and ‘Build Back Better’ as we emerge from the Covid pandemic, East Anglia must seize the massive opportunities ahead in order to unlock its full potential.
That’s why I have signed a joint letter to the Chancellor (with a number of other MPS from Norfolk, Suffolk, Cambridgeshire and Essex) calling for him to grant Freeport status to the Freeport East bid.
Our region, and particularly Norfolk, is ideally placed to play a frontline role in the ‘Green Revolution’ that the country is embarking upon, and this bid seeks to enhance that further by bringing even more jobs, skills, investment and prosperity – all the while driving us towards achieving our national Net Zero targets.
Not only could it significantly ‘level up’ the prosperity for the East of England, it could also host the UK’s first Green Hydrogen Hub – an enormous step forwards as we look to generate Clean, Green Energy and begin the process of decarbonising the highly polluting shipping industry.
With the southern North Sea about to become the world’s largest hub of offshore wind energy (and I am leading efforts alongside several Norfolk and Suffolk MPs to ensure that we have a proper integrated Offshore network for these new wind farms – to read more, please click here) and major nuclear power projects nearby, a Freeport on the Suffolk coast would create another national centre of technical excellence in East Anglia – alongside the likes of the Norwich Research Park, the Hethel Engineering Centre and the developing Food Enterprise Park at Honingham (all around Mid Norfolk), as well as the University of Cambridge and BT’s Adastral Park more widely.
The Department for International Trade already recognises the East as a High Potential Opportunity Location and, rest assured, I am committed to working with colleagues on projects to ensure that we make the most of this – to get the very best for our people and businesses AND help deliver the ‘Greener’ future we all need.
See the full letter to the Chancellor below.

Having worked in business for over fifteen years before becoming Mid Norfolk’s MP, and having served as a Business Minister, I know just how important local businesses are to both our local and national economy. They really are the engine that drives our country forward!
That’s why I remain committed to continuing my efforts to speaking up for Business locally and in the House.
I was delighted to join in with the Attleborough and Snetterton Business Forum this week to talk through the Town Plan Partnership and Post Covid Regeneration.

This week the PM set out the Government’s Roadmap out of Lockdown.
I’m sure that for you - as for us all - it couldn’t come too soon. Lockdown has been hugely difficult & damaging for so many households, families, pubs, shops & local businesses & communities.
So, the news of a route out is hugely welcome.
But it’s also clear that the longer-term damage and legacy of the Covid pandemic will require some big bold measures from Government in London and here locally to ensure a strong post-Covid Recovery
That is why I was delighted to have been approached by Radio Norfolk with my thoughts on the roadmap out of Lockdown.
There will be lessons to learn from this terrible global pandemic, and it just goes to show how infectious diseases can damage economies on such a large scale, and how the vaccination programme we have in place is so important for that economic recovery.
Rest assured I am working closely with the LEP and councillors to do all I can to ensure Mid Norfolk gets what it deserves by way of financial help for businesses to survive and grow, and High streets to thrive for our future generations. This is a huge opportunity to re-invest in skills training.
If we get this right the post-Covid Recovery is an opportunity to embrace a seriously bold & ambitious vision for Norfolk,
For more information on the Government Roadmap please click : here
To read more about how I believe Norfolk can become a centre of science and innovation post-Covid, please click: here
It was so good to read that the ongoing works at Attleborough Train Station are to resume as part of the plan to increase the capacity for parking to 86 cars, as well as spaces for motorcycles.

I was particularly glad to see there will also be an installation for electric car charging in preparation for future, increase use by the public. This is going to be such a vital part of our transport sector long term – so important that we get ahead of it now!
As we look towards a greener Net Zero future in which we should all be embracing healthier lifestyles, projects like this are crucial. We must put People and Places at the heart of the vision for public transport and accessibility for all, and as many people as possible at once, is the first ingredient of that.
Our local Train Stations can be hubs of activity – for Transport, Business and Socialising. They should be ‘gateways’ for our communities, allowing easily travel around the region, as well as places for local businesses to come together, network and utilise ultrafast broadband – and for people to come together for a coffee and a catch up.
That’s why I continue to do all I can to promote the potential of our local stations, and why I am working with local councillors, the Town Council, our district and county councils and the likes of Network Rail and Greater Anglia – encouraging them to work together on a longer term vision for these vital assets.
Well done Greater Anglia! I look forward to seeing the progress made soon.
To read more about the plans at Attleborough station please click here
Although not opposed to the principle of having a new Weather Radar Tower in my constituency, since day one I have had concerns about the Met Office proposals to build one at the Anglian Water site in Old Buckenham.
At the start of 2020, I supported local concerns about the potential impact of the Tower on the nearby Old Buckenham Airfield (a major tourist asset in our part of the world) and offered to work with the Met Office to find another, more suitable location in Mid Norfolk. Sadly, the Met Office did not take up that offer – and the application was not successfully carried through.
I have now received a wave of correspondence from concerned residents in the village in light of the new application that the Met Office have tabled.
While they have heeded the concerns of many about the potential impacts on the Airfield and amended their proposals accordingly, the massive physical and visual concern of the tower on this very rural area is a source of much worry. Furthermore, it has been discovered that there are highly endangered bat species in the area – and there is a desire to do more research into any likely impacts that the weather radar may have upon them.
That’s why I have written the letter below to Breckland Council – highlighting my concerns and once again offering to help the Met Office find an alternative site that is more acceptable to the local area.
Please be assured, I shall continue to follow this closely.
Big news! A High Court judge has overturned the planning permission given to the Norfolk Vanguard Offshore Windfarm – which would have seen underground cabling from the North Norfolk coast to a new substation the size of Wembley Stadium at Necton.
While I am a major advocate for the Government’s ‘Green’ agenda and the drive for Offshore Wind Energy (which presents a massive opportunity for our region), it has become increasingly clear that the current approach to delivering this crucial infrastructure is not fit for purpose
For all of those of us that love and appreciate our beautiful landscape here in the East, it is clear that the chaotic “free for all” approach currently being taken cannot be allowed to continue. With proper debate and an overall strategic plan, we can ensure that the huge environmental and planning impacts currently being faced are significantly reduced – all the while delivering the much needed offshore renewable wind energy that our future economy and society will need.
We owe it to the generations who will come after us to get this right and leave our county as beautiful as we found it.
Rest assured I am firmly committed to continuing the fight for a proper strategic plan both locally and nationally.
To read more about the Norfolk Vanguard decision, please see the EDP article here
To read more about my work on Offshore Wind, please visit my website here

One of my key missions since becoming the MP for Mid Norfolk has been supporting our local town and village pubs – which play such a key role at the heart of our communities and rural heritage.

That is why I was delighted to hear the news that planning permission has been given for works to proceed.
A huge congratulations to all the team, Great work.
The campaign has been a marvellous success this year and I hope to visit The White Swan pub in the newly renovated and reopened for the local community this year. Hopefully I can pull a pint or two soon.
To see some of my previous work on the campaign, please click here
Having left my entrepreneurial career to stand on a platform of restoring trust in our broken politics after the MPs expenses scandal, I take compliance with parliamentary and ministerial rules extremely seriously. Please see below the chronological chain of ACOBA’s recent findings on my work with Aerosol Shield last year- including my statements and my latest letter to ACOBA.
Update 16 Feb 2021
Following the most recent letter from Lord Pickles, please see here my response to ACOBA, dated 16 Feb 2021
Update 1 Feb 2021
My latest correspondence with ACOBA, dated 28 Jan 2021
---
27 Jan 2021
I am appalled that my pro-bono work helping identify technologies to help the Government’s Covid crisis is being misrepresented as a breach of ACOBA rules.
Last March and April, there was a National call from Ministers for a national effort to source any technology to help with the Covid emergency: from ventilators to PPE. With a background in medical technology, I did all I could to help - including securing a charitable gift of disposable PPE for frontline healthcare workers in Norfolk.
Although this had nothing to do with my previous role in Government at the Department of Transport, but my professional background in Life Science technology before coming to Parliament, I contacted ACOBA to seek advice and make sure there could be no confusion.
They were clear that I did NOT require ACOBA approval for this - only for any ongoing long term commercial work which might transpire which fell within ACOBA’s remit which is to ensure Ministers do not exploit insights or influence gained through their previous Ministerial experience through commercial work within two years of holding office.
Aerosol Shield had an innovative new idea for a much stronger face shield which could protect frontline NHS staff. Along with other proposals, I helped ensure Ministers were aware of this new equipment which could potentially save thousands of lives.
Sadly Aerosol Shield were turned down by NHS procurement.
—
Two months later, the founding medics then decided to try and raise money from commercial technology investors and asked me if I would help them with their plans for that, given my professional career background in this sector.
In June, I did a short piece of consulting work with Aerosol Shield for which I invoiced for £5k, and fully declared in the Register of Interests.
On the basis of ACOBA’s advice, because it had nothing to do with my previous role at DfT, and was not an ongoing piece of work, I did not believe it needed clearing with ACOBA.
ACOBA have themselves admitted to me that the guidance wasn’t clear and apologised to me to me for that, and confirmed that I have ‘done everything asked in responding and the matter is closed from ACOBA’s perspective’.
Now no longer a Minister I am involved in a number of Not-For-Profit and charitable projects - from The Bridge of Hope set up in memory of my late father, to The Big Tent Foundation, The Norfolk Way Festival, and an international Health Resilience Commission, none of which involve exploiting any Ministerial benefits, but all of which I am discussing with ACOBA to avoid any further confusion.
__
November 2020
