Further to my webstory last month (see below – dated 18th September 2023), I am delighted to share news from National Highways that the speed limit at the dangerous A47 Necton/Dunham junction will be permanently reduced from 50mph to 40mph as part of the ongoing construction works – subject to the completion of statutory processes.
Fantastic news!
Having helped secure the safety improvements currently underway, this latest progress is very positive indeed and a further boost for local road safety.
Rest assured however, my work to improve road safety across Mid Norfolk continues. I will continue to lobby National Highways and others on blackspots such as those in Guist and at the Draytonhall Lane A47 Junction.
To see more about my ongoing work to improve road safety and infrastructure in Norfolk, please visit my campaign page here
To see more about my historic work with Necton Parish Council and Cllrs Nigel Wilkin and Mark Kiddle-Morris on the A47 Necton/Dunham Junction, please see my previous webstory below.
The A47 Necton/Dunham junction has long been a source of major concern for those that have cause to use it.
That’s why I was delighted to help secure a commitment from National Highways for improvement works at this dangerous location (having long campaigned with local district, county and parish councillors to that end), and why I am thrilled to now have confirmation that the works will finally get underway next Monday (25th September 2023).
Another pledge successfully delivered!
The £2.5 million improvement project is forecast to take place over five months, with most of the work taking place on weeknights between 8pm-6am – when traffic flows are typically lighter. Both sides of the main A47 Necton/Dunham junction (at Tuns Road and Dunham Road) are set to be widened, with improved drainage and road markings also being put in place. As a result, it should be much safer to access and depart each side of the junction – with visibility significantly improved too.
Having pushed hard for safety improvements at this key junction, these works are a significant step forward. There is still more to do however as I work with the community to push for a permanent speed limit reduction from 50mph to 40mph and support further conversations about additional safety improvements that can be made at this location.
It was a pleasure to hold another site visit with Cllr Nigel Wilkin recently, at which we filmed a short video to update local constituents on the works that are about to commence. Please see below.
Rest assured, I will continue to work hard for further A47 safety improvements (including the also horrendous Draytonhall Lane Junction at Scarning), as well as towards the longer term aim of full A47 dualling.
To see more about my campaign to improve the A47 Necton/Dunham junction, please click here
To find about my wider campaign to improve Norfolk’s roads, please click here.

My statement following Hamas’ attack on Israel.

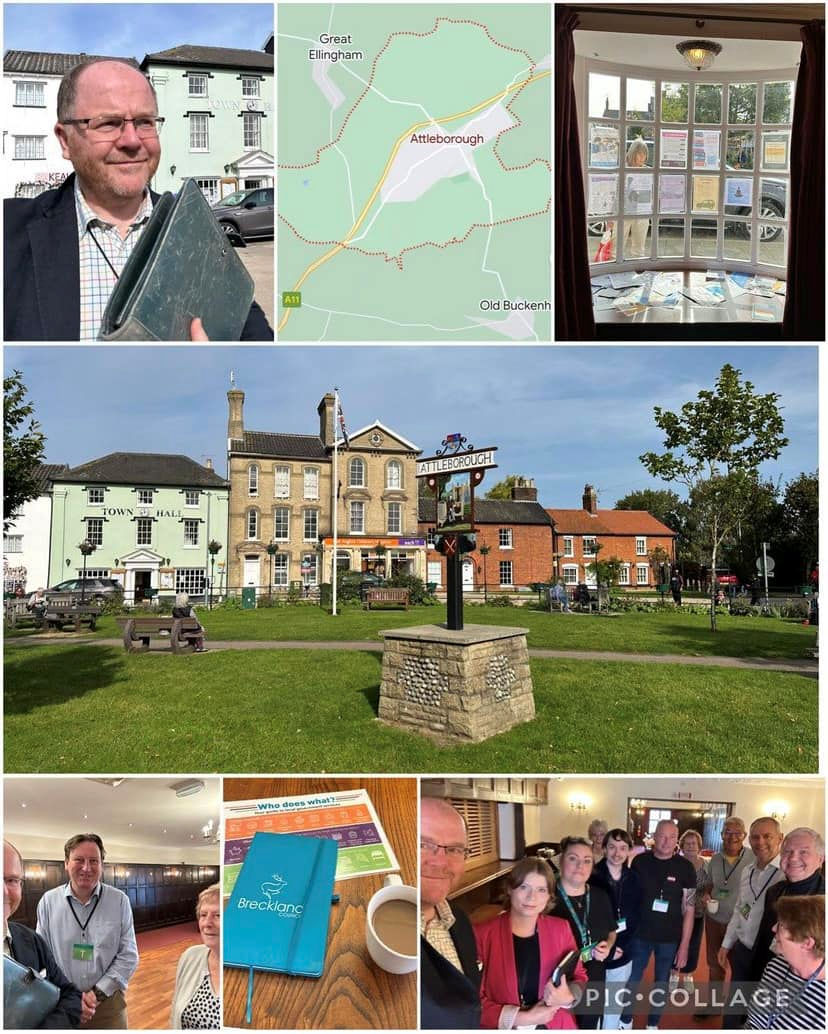
Great to do a drop-in Community Saturday Surgery in Attleborough this past Saturday morning with local councillors from Attleborough Town Council, Breckland Council and Norfolk County Council, talking to residents about:
- The Town Plan
- Urgency of the new link road as 1000’s of new houses being built to prevent worsening traffic gridlock
- #Attcare Health plan for local community health facilities
- Need for a bank for cash for High Street businesses
and much more…
I look forward to posting further updates on all of this work in the weeks and months ahead.
As has been widely publicised in recent years, the East is at the forefront of the offshore wind revolution that is already strengthening our UK energy resilience and driving forward the decarbonisation of our energy network.
That’s why, having been a leading figure over several years calling for a proper offshore solution for delivering this crucial Offshore Wind Infrastructure, I was proud to co-sign an OffSET MP letter to the new Secretary of State last week – in which we reiterated the importance of proper community consultation around the question of offshore infrastructure versus onshore pylons.
The next round of consultation cannot begin until the findings of National Grid ESO’s review into offshore options are known and communities have been fully engaged.
While particularly relevant to the East Anglia Green proposals, this issue is very much connected to the wider campaign for a proper solution – one that would save the British taxpayer billions over the coming decades, minimise environmental and community disruption and in fact enable to the UK to accelerate its offshore wind production in the years to come.
We, the OffSET MP collective, have also asked for an urgent meeting with the Secretary of State too.
Rest assured, I am remain committed to speaking up for the people of Mid Norfolk on this issue – and I hope to be able to provide further updates in the not too distant future.
To learn more about my campaign work on this issue, please visit my website here.

Connectivity provides a lifeline to rural counties like our own.
That’s why I am a vocal supporter of the campaign to dual the A47, and am proud to have played my role in getting the A11 dualled.
See my recent column for the Dereham Times below.

Local communities, as well as those moving into new developments, need to know that they can access the local services they need, when they need them.
That’s why, throughout my time as a local MP, I have consistently made clear (locally and to ministers) my belief that new developments in our rural towns and villages must be sustainable, accompanied by the necessary infrastructure and services required to support both the new development itself and the pre-existing community. Health provision is an integral part of that.
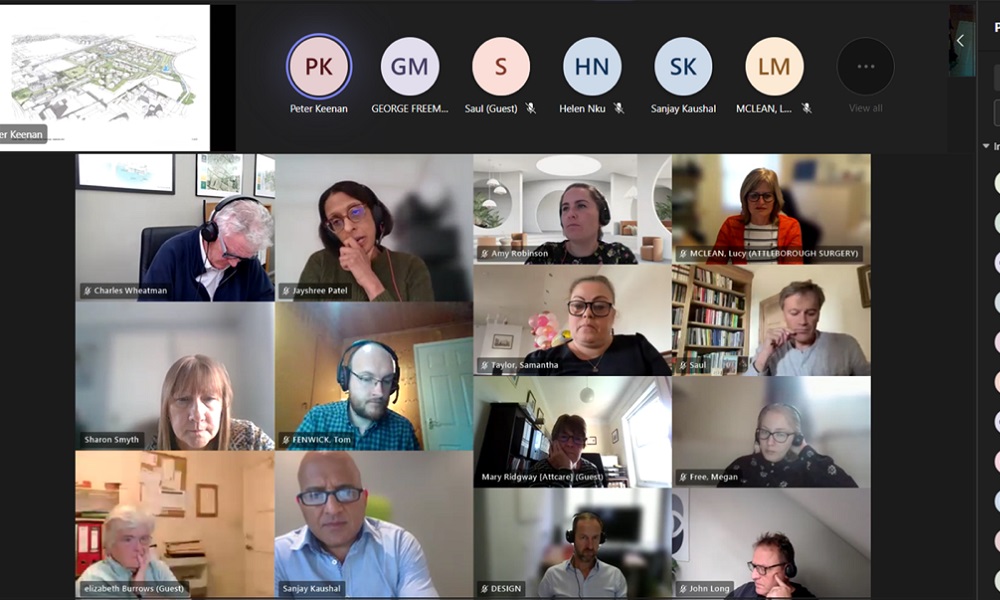
On Friday, I welcomed the chance to chair the latest ATTCARE virtual summit – which aims to address exactly that issue. In attendance were local councillors and representatives from ATTCARE, Attleborough Town Council, Attleborough Surgeries, Norfolk Community Health and Care Trust, Norfolk County Council, Breckland Council, Ptarmigan, Homes England and Castlemeadow Care.
The conversations were positive once again with the sub-working groups reporting back on the progress they have made to date, as well as on their work moving forward. The news that a modular extension will now go ahead at the Attleborough Surgeries’ Station Road site is also very welcome. Although only a temporary solution, the extension will allow the practice’s dedicated team of staff to tap into additional resources, expand capacity and strengthen the services they can provide patients. Work should begin imminently. (See more in the recent EDP article here)
A number of follow-up- actions are being pursued and all those gathered remain committed to working collaboratively. Rest assured, I am determined to do whatever I can to support the work of ATTCARE and their partners on this work. There remains much work to be done but, together, we hope to deliver the short term boosts the town wants to see, as well as the longer term solutions that will ensure Attleborough has the long term health and social care services it requires to sustain the community.
To see more about my previous work on this issue, please click here and here
I look forward to providing further updates in due course.

Local pubs are so often the beating hearts of our towns and villages. Not only are they vital for our local economy, they do so much great work in their communities and help generate the famous rural community spirit that our beautiful part of the world is renowned for.
That’s why, throughout my time as the local MP, I have actively promoted and visited dozens of our rural pubs – the Beeston Ploughshare, The White Swan at Gressenhall, Darby’s in Swanton Morley, The Crown at Great Ellingham, The Railway Tavern at Dereham and The Lodge at North Tuddenham to name just a few. We are blessed that Mid Norfolk is home to some of our county’s finest establishments!
However, as a former Business Minister and small business owner myself, I know how tough it can be for those running our local pubs. Like so many businesses in recent times, our rural pubs have faced the perfect storm of increased energy prices and the cost of living crisis, which have meant far higher operating costs and much more limited disposable income for the customers that frequent them. Compounded by the impacts of the pandemic beforehand, the pub sector is going through a very hard time.
That’s why, whenever I can, I seek to promote this wonderful sector – and why I frequently speak out in support of our town and village pubs in Westminster.
Rural communities depend on active community services to stay thriving and vibrant. Pubs are a vital part of that.
To learn more of my work supporting this crucial sector over the years, please visit my campaign page here.

Local pubs are so often the beating hearts of our towns and villages. Not only are they vital for our local economy, they do so much great work in their communities and help generate the famous rural community spirit that our beautiful part of the world is renowned for.
That’s why, throughout my time as the local MP, I have actively promoted and visited dozens of our rural pubs – the Beeston Ploughshare, The White Swan at Gressenhall, Darby’s in Swanton Morley, The Crown at Great Ellingham, The Railway Tavern at Dereham and The Lodge at North Tuddenham to name just a few. We are blessed that Mid Norfolk is home to some of our county’s finest establishments!
However, as a former Business Minister and small business owner myself, I know how tough it can be for those running our local pubs. Like so many businesses in recent times, our rural pubs have faced the perfect storm of increased energy prices and the cost of living crisis, which have meant far higher operating costs and much more limited disposable income for the customers that frequent them. Compounded by the impacts of the pandemic beforehand, the pub sector is going through a very hard time.
That’s why, whenever I can, I seek to promote this wonderful sector – and why I frequently speak out in support of our town and village pubs in Westminster.
Rural communities depend on active community services to stay thriving and vibrant. Pubs are a vital part of that.
To learn more of my work supporting this crucial sector over the years, please see the webstories below and my Facebook page here.
Following the Prime Minister’s announcement of a Net Zero reset, please see my statement below setting out my thoughts.


Rural schools, much like our rural pubs, shops and churches, play a crucial role in our local communities and rural way of life.
That’s why, having grown up in East Anglia and always been a firm champion of that rural way of life, I’ve been a vocal supporter of keeping our smaller rural schools open wherever possible. It’s also the reason I very much understand why there is so much anger and concern at the news that the Diocese of Norwich Education and Academies Trust (DNEAT) is now consulting on proposals to cease maintaining the Weasenham Primary Academy site and instead merge the school with Brisley Primary Academy from 1st January 2024 – citing school size, declining pupil forecasts and associated financial viability as the reasons for doing so.
(The consultation is also looking at changing the catchment area of the parish of Weasenham so that Massingham School becomes the catchment school from 1st January 2024).
Full consultation details can be found here:
Over recent days, I’ve been in contact with the WeasenhamParish Chair, local county councillor Mark Kiddle-Morris and a number of people from the local community with concerns.
While, in my role as a local MP, I am limited in the direct influence that I have in such matters and must respect the proper due processes in place, as ever, I always strive to do what I can to help ensure my Mid Norfolk constituents’ voices are being at the highest levels.
That’s why I am highlighting the consultation process here.
If YOU have views on the proposals, PLEASE do take the time to have YOUR say – by:
• Emailing info@dneat.org (quoting Weasenham as the ‘Subject’ of your response)
• Writing to Weasenham Church of England Primary Academy Consultation, DNEAT, Orchard House, Hall Lane, East Tuddenham, Norfolk, NR20 3LR
Before 5pm on Friday 6th October 2023.
The stronger the local voice, the better chance there is that DNEAT will take note.
IF, however, the consultation feedback alongside the population demographics for the Weasenham area proves insufficient in justifying the village having its own school in future, I believe the Alternative Plan must work for parents and pupils. There needs to be an outcome that suits the community best. We want to avoid young children having to be bussed or driven by parents over long distances.
In that event, I remain committed to working with all local parties, but especially the local community, to help get the best possible outcome for local people. At this stage though, I am determined to help local constituents engage in the consultation process and have their say.
Please do take the time to have YOUR say.
To see more on my work supporting our local rural schools, please visit my website here

