

How do we build an integrated Health & Care system in Norfolk combining:

A47 Alliance meeting

Enough is enough. Back in 2014 I brought the then Prime Minister to Mid Norfolk who committed £300m for Phase 1 of full dualling of the A47 and improvements between North Tuddenham and Easton. Six years later and Highways England have still not started these works. Meanwhile, people are dying on our roads and business investment is on hold. It is time for action.
I was, therefore, delighted to join the A47 Alliance again this past Friday with fellow Norfolk MPs Jerome Mayhew and Duncan Baker and agree to:
- Take all MPs to lobby the new Chancellor
- Convene a Business Partnership Summit
- Volunteer to lead a new Task Force on how we can show private investment to boost the business case
Although these infrastructure improvements will represent massive progress for our area, it is also vital that we ensure that a proper strategic plan is put in place to prevent any rat-running problems through local communities. Back in October, at the request of a number of local Parish Councils, I attended a public meeting at St Peter’s Church in Kimberley to hear in greater detail their concerns about this potential increase in rat-running through our rural communities. It was decided that a local Taskforce would be created to co-ordinate the villages affected and work with local councillors, county and district officers, Highways England officials, the A47 Alliance and countryside groups to oversee the development of a proper Plan around the works. You can keep up-to-date with the work of the Task Force via the campaign on my website here: https://www.georgefreeman.co.uk/content/a47-rat-running-campaign-and-taskforce-0
While the Taskforce wants EVERYONE to submit their views to the dedicated Norfolk County Council and Highways England consultations, it would also like to ask members of the public to share their views with the Taskforce directly as well. To submit your views to the Taskforce, please email them at: A47taskforce@outlook.com
On another busy weekend of constituency meetings pushing a number of key local projects with local Councillors, community campaigners and local businesses I was pleased to meet with Andrew Proctor, Leader of Norfolk County Council and the Deputy Leader, Graham Plant, at County Hall.
We discussed a range of issues relating to Mid Norfolk and the wider County and I look forward to continuing to work closely with the leadership team.

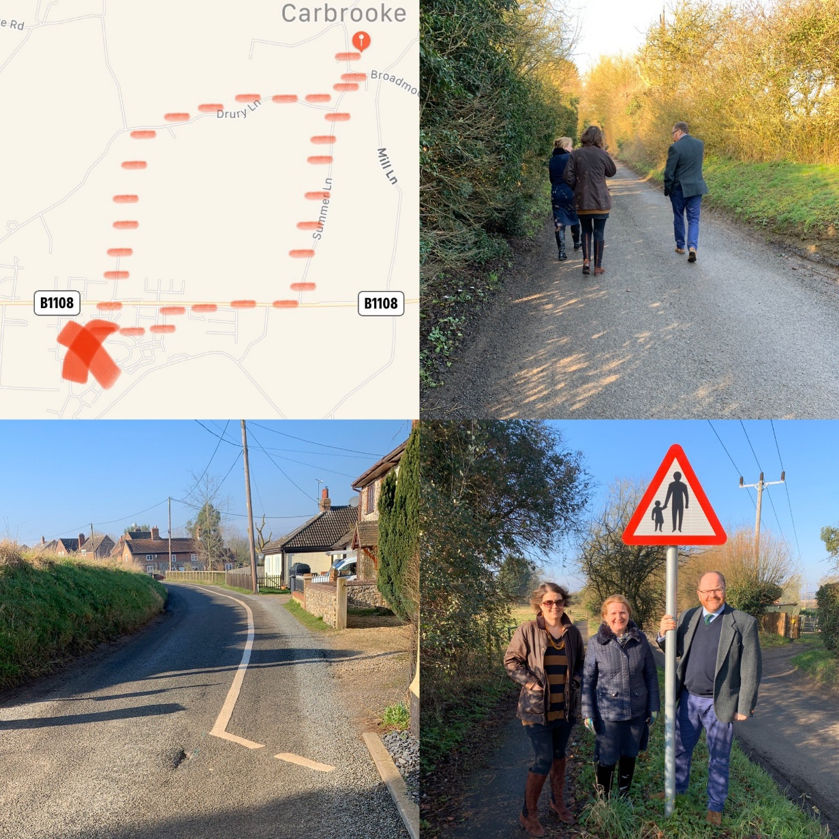
Friday’s constituency day began at Carbrooke - meeting with parish, district and county councillors as well as school governors to discuss the poor footpath facilities for children waking from Blenheim Grange to the village school. It is simply not acceptable for primary school children to have to walk 2 miles down a narrow country lane with no footpath and fast traffic to get to school.

Mid Norfolk is fizzing with many local community events taking place throughout the year from vintage days to school fetes to player’s group productions.
One of the privileges of my role is being able to use my position to support great causes to help promote local initiatives. If you are part of a Mid Norfolk group that is looking to promote your community event or cause then do feel free to email me with all the details, including date and times, as well as with relevant imagery or links and social media handles so I can share on my social media platforms and website.
Please note, I am only able to share details of events taking place in the Mid Norfolk constituency or in Norfolk by a group based in the constituency. Due to the number of events, it may not be possible to share details of every request or across all platforms.


Although I am no longer a Minister at the Department for Transport, I continue to take a keen interest in transport matters – especially ones which have a direct impact on Mid Norfolk.
A fundamental of a good transport system is ensuring that EVERYONE has access to our railway stations, and for too long those who are disabled, or with bicycles or prams, have struggled to access Platform 2 of Wymondham Station. Many have to travel to Norwich or Attleborough to use the disabled facilities there in order for them to be able to switch sides and continue with their journeys. This is clearly unacceptable.
That’s why I am pleased that the government is taking access issues seriously across the country, with £20 million of funding being provided for accessibility improvements. I am hoping that our bid submitted via the Wymondham Station Task Force with Greater Anglia and South Norfolk District Council for disabled access facilities at Wymondham Station will be successful.
In the meantime of this being considered, I continue to work closely with the Task Force of the relevant parties and will continue to push for access for all.
You can read more about the government announcement here.

It was a privilege to meet with the brilliant Norfolk-based military charity Scotty’s Little Soldiers at a reception in Parliament this past Tuesday, alongside fellow Norfolk MPs.
Scotty’s Little Soldiers is dedicated to supporting the children of men and women lost while serving with the British Armed Forces.
The charity’s assistance is divided into 3 distinct programmes:
Smiles - offers the children that opportunity to smile again through a wide range of fun activities and gifts. This includes holiday breaks, group events, special experiences and gifts at difficult times of the year.
Support - aims to assist with the more emotional side of bereavement and includes access to professional counselling and a family support network.
Strides - is designed to help with the charity’s beneficiaries’ personal development and includes a range of activity and educational grants.
You can read more about each of the assistance programmes by visiting their website: https://www.scottyslittlesoldiers.co.uk/
I look forward to working with the charity in the coming months and years ahead.

